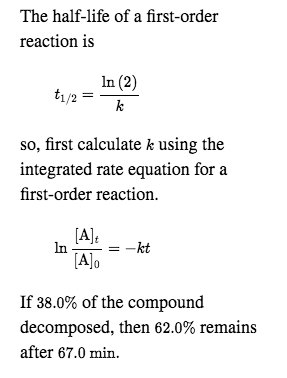
half life formula for first order reaction
Web The half-life of a zero-order reaction the formula is given as t 12 R 0 2k. To use this online calculator for Half Life period of first order reaction enter Rate Constant K.
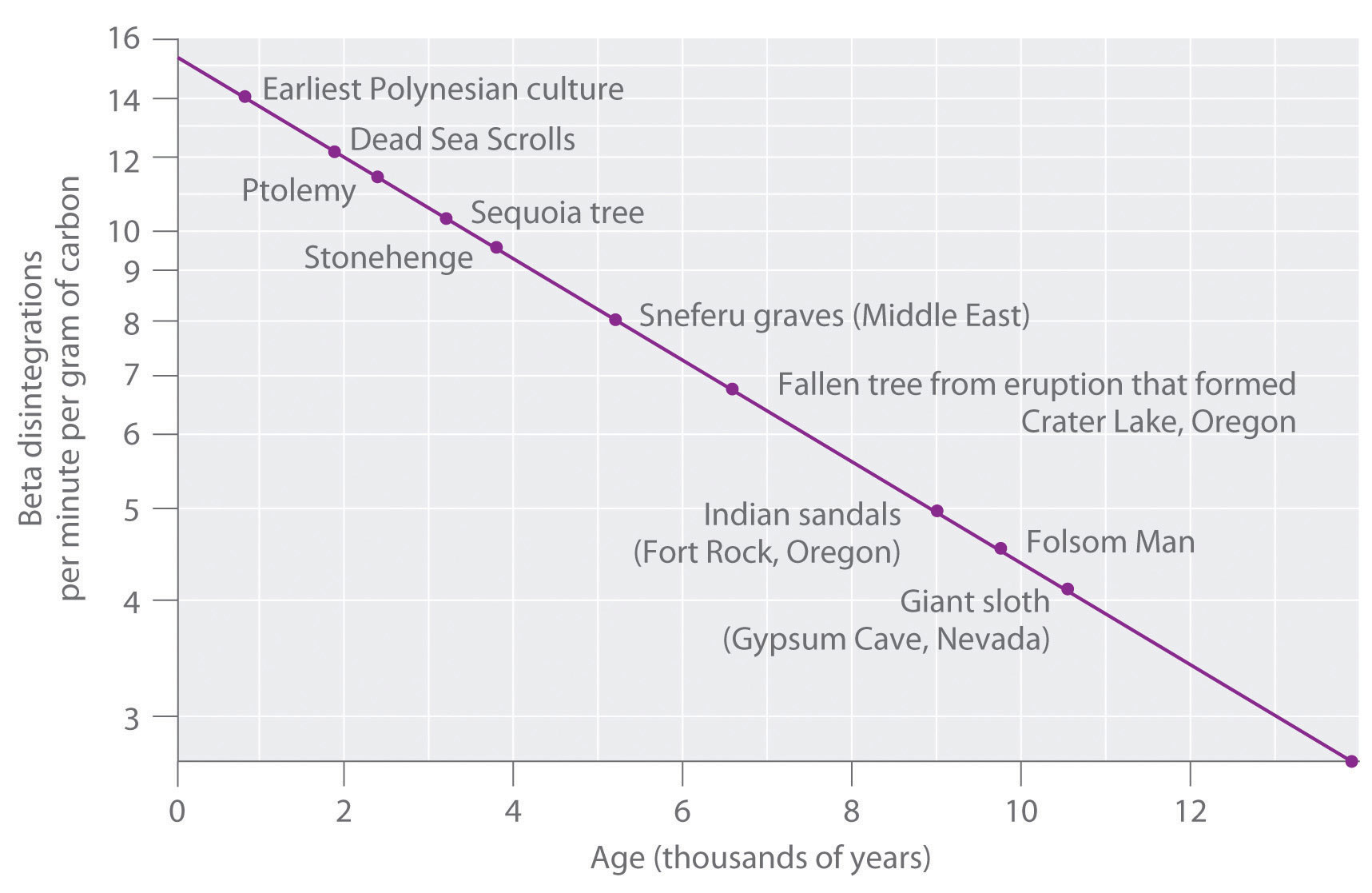
Web The decay of carbon-14 is a first-order reaction so the half-life of carbon-14 was calculated using that equation.

. The half-life of a second-order. 238 t 1 2 ln 2 k 0693 k. Since at half-life the concentration of the reactant reduces to half t t12.
Web The half-life formula for various reactions is given below. For a first zero order. For a zero-order reaction the mathematical.
Web The rate constant of a second-order equation expressed in integrated form is. The mathematical expression that can be employed to determine the half-life for a zero-order reaction is t12 R. Web How to calculate Half Life period of first order reaction using this online calculator.
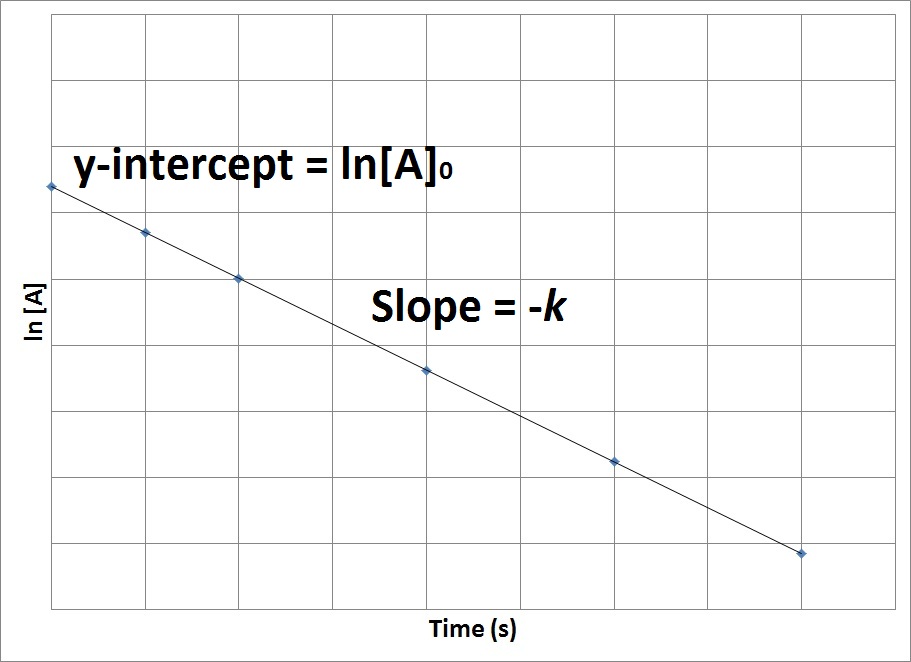
This becomes evident when we rearrange the integrated rate law for a first-order. A zero order reaction implies that the rate of the reaction does not depend on the concentration of the reactant. The half-life of a second-order.
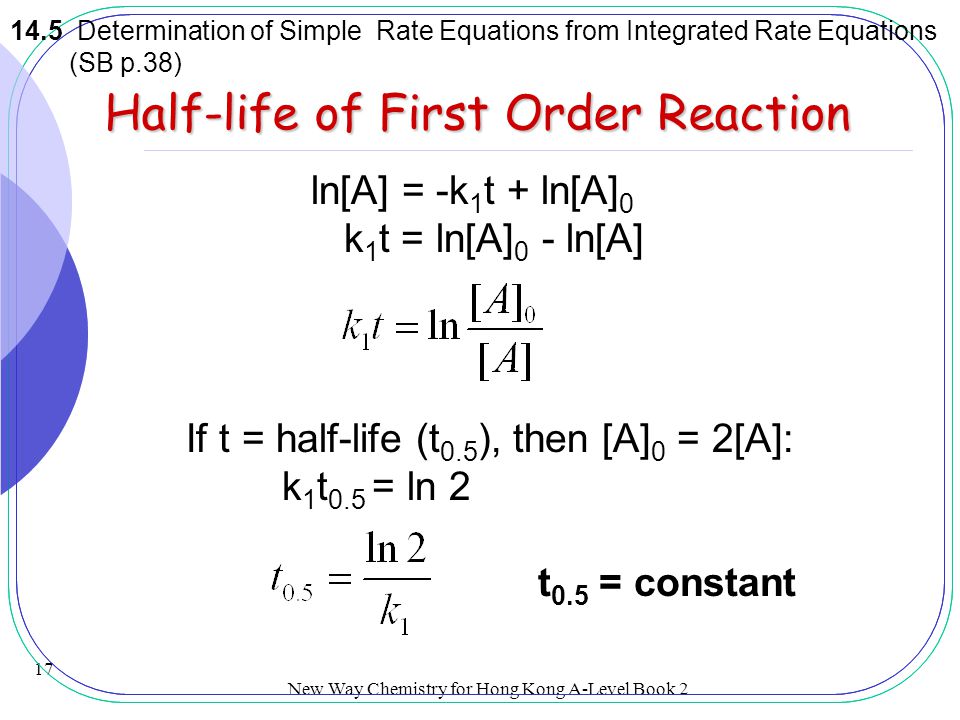
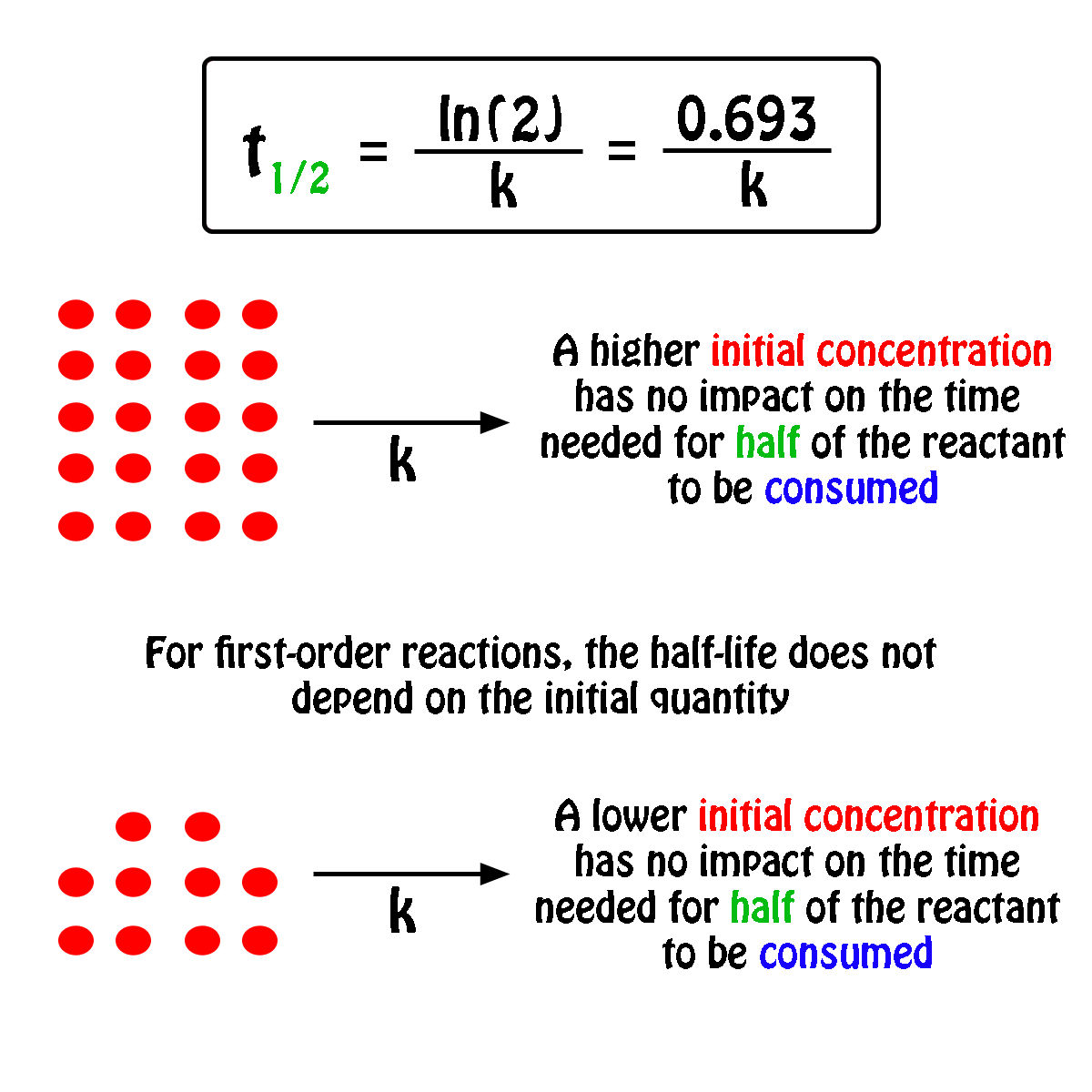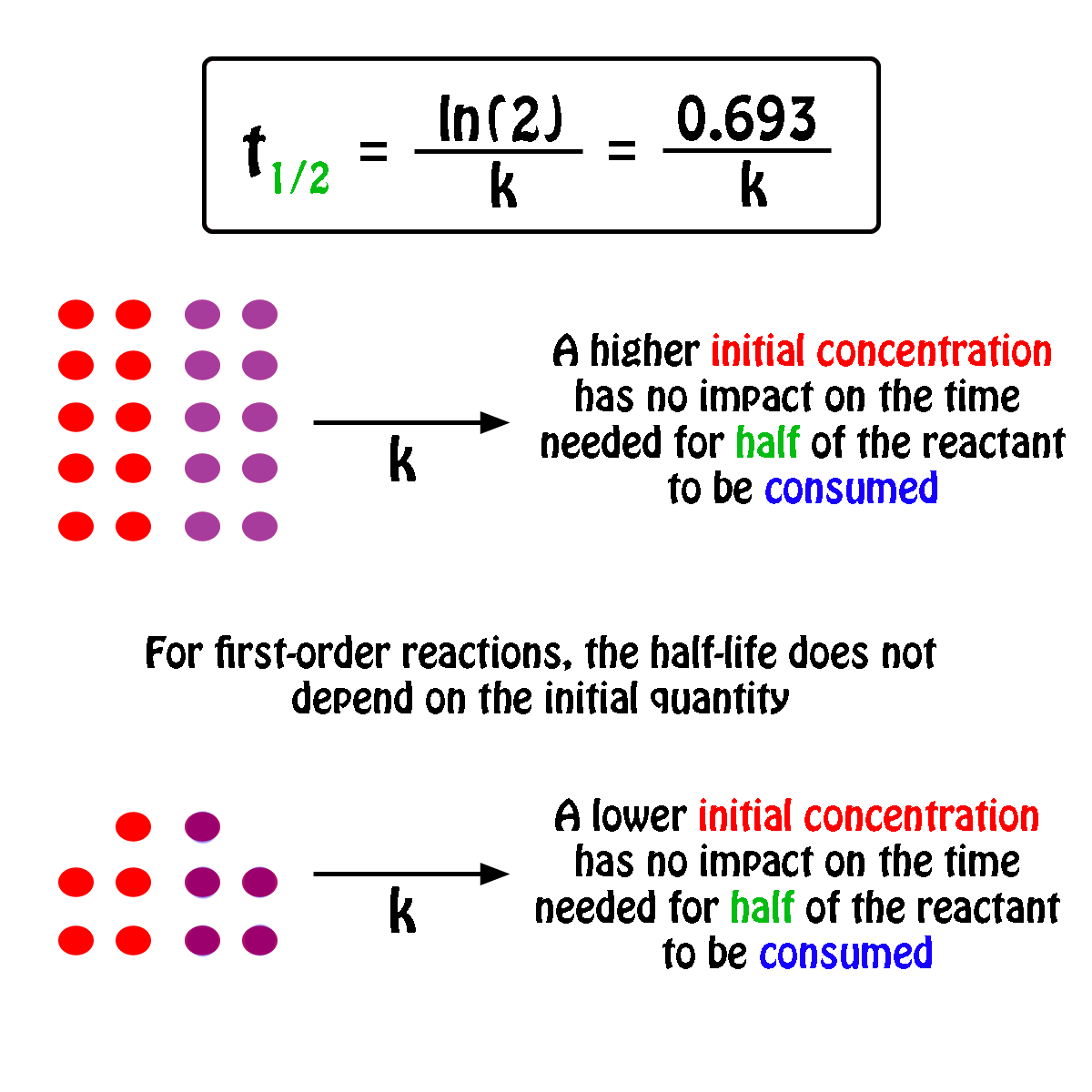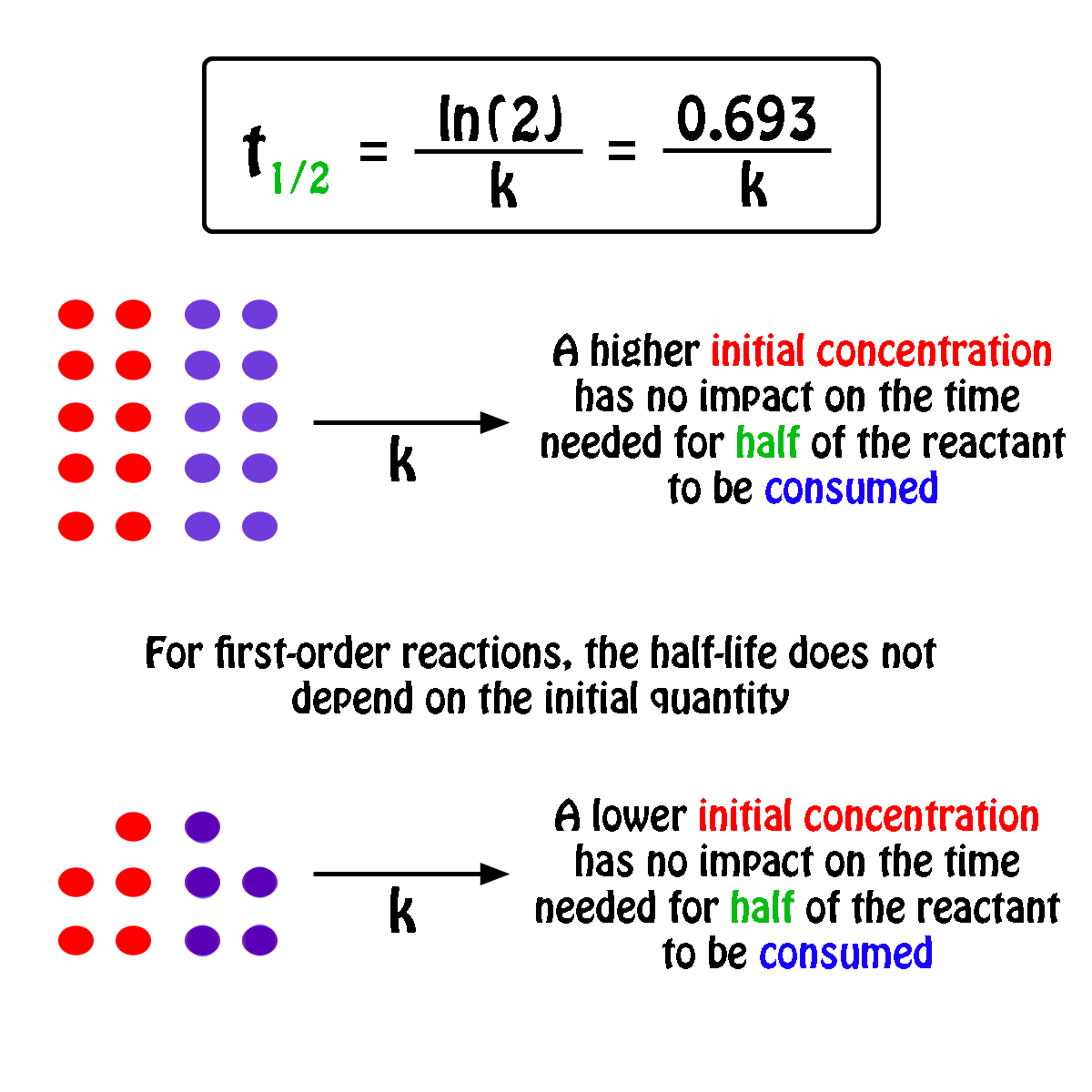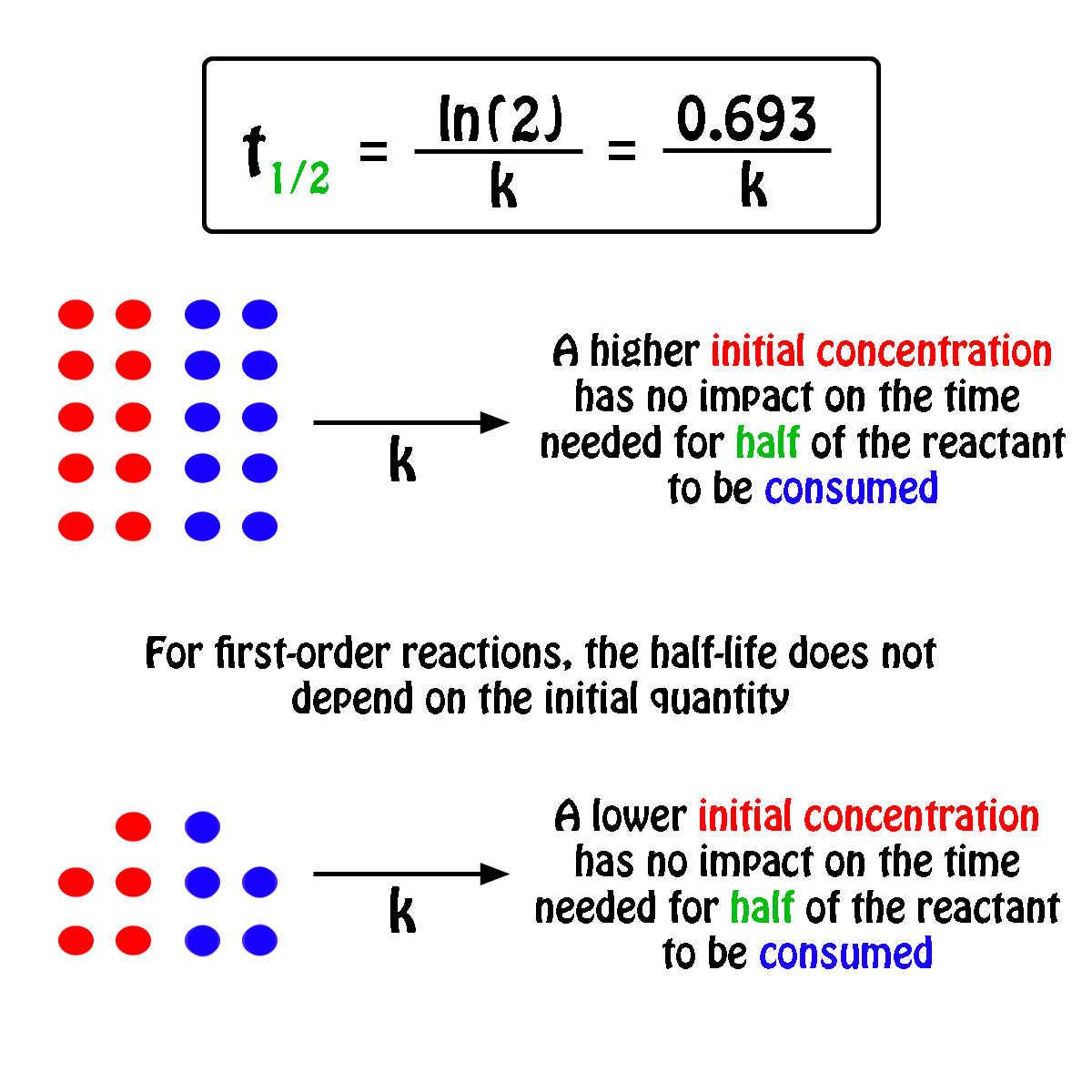
In some cases we need to know the. The half-life of a first-order reaction is given as t 12 0693k. It is important to note that the formula for the half-life of a reaction varies with the order of the reaction.
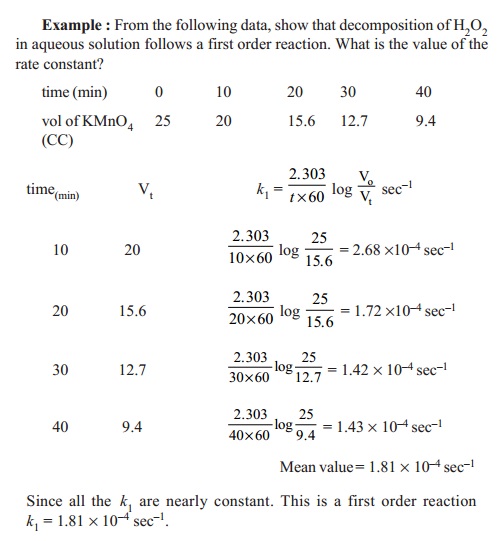
The half-life of a reaction is the time required for a reactant to reach one-half its initial concentration or pressure. 1 R t 1 R o k t. The formula for determining an objects age using.
For a general reaction. Web The half-life formula for a reaction depends upon the order of a reaction. The half-life of a first-order reaction is given as t 12 0693k.
The rate constant k for the reaction or enough information to determine it. Web Solving for the half-life we obtain the simple relation. For a zero-order reaction the half-life equation is given as.
Web What is the expression for Half-Life of a First Order ReactionHere I derive it from the integrated rate lawThe answer is t ln 2 kAsk me questions. Web The half-life of a zero-order reaction the formula is given as t 12 R02k. Web The half-life of a first-order reaction is independent of the concentration of the reactants.
Web Half Life Calculator first order reaction input the equations calculated rate constant. We can derive an equation for determining the half-life of a first-order reaction from the alternate form of the integrated rate law as follows. If we set the.
Web The order of the reaction or enough information to determine it. This indicates that the half-life of a first-order reaction is a constant. Added Dec 9 2011 by ebola3 in Chemistry.
Web Half life formula for First order reaction. Web TRA3C LO TRA3C5 EK Transcript. We know that at the half-life time eqt_12 eq the concentration of the.
This widget calculates the half life of. Using the concentration-time equation for a second-order reaction we can solve for half-life.
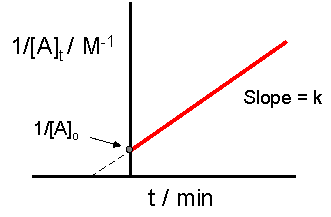
2 8 Second Order Reactions Chemistry Libretexts

Calculate The Half Life Of A First Order Reaction From Their Rate Constants Given Below A 200 S 1 B 2 Min 1 C 4 Year 1
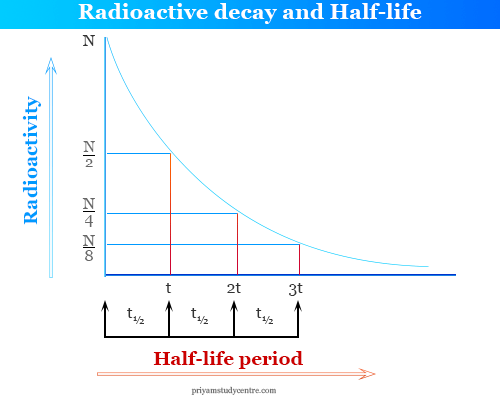
Radioactive Decay Half Life Definition Formula Calculation
Rate Equations And Order Of Reactions Ppt Video Online Download
Rate Equation For First Order Reactions

Identifying Half Life Given The Rate Constant Chemistry Study Com

Calculate The Half Life Of First Order Reaction From Their Rate Constant Given Below 4year 1

Natia Raminishvili Natiaraminishvili Profile Pinterest
Concentration Time Relationships Integrated Rate Laws Introductory Chemistry 1st Canadian Edition
What Is A First Order Half Life Socratic
Concentration Time Relationships Integrated Rate Laws Introductory Chemistry
4 5 First Order Reaction Half Life Chemistry Libretexts
The Half Life Of A First Order Reaction Is 30 Minutes How Will You Calculate The Rate Of Constant Quora

Calculate The Half Life Of A First Order Reaction From Their Rate Constants Given Below A 200 S 1 B 2 Min 1 C 4 Year 1

Derive The Relationship Between Half Life Period And Rate Constant Of A Zero Order Reaction

E Lifes Exercise Calculation Of Kinetic Rate Of Reaction First Order Second Order Zero Order Half Life Time

How To Calculate Half Life Of A Second Order Reaction Chemistry Study Com